
COVID-19 and Respiratory Viruses PCR Nasal Collection Instructions
Consistent with O. Reg. 671/92 of the French Language Services Act, test ordering and instructions on this page is only available in English because it is scientific or technical in nature and is for use only by qualified health care providers and not by members of the public.
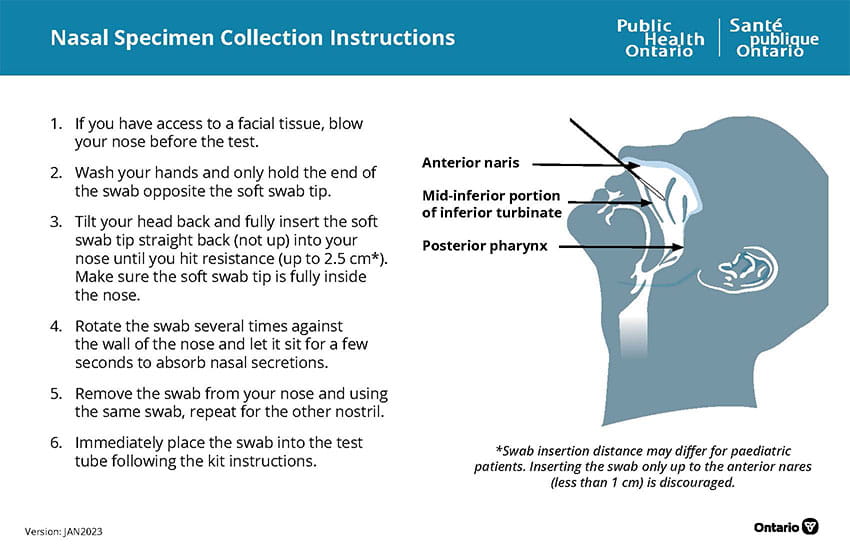
Click here to download the visual instructions of the Nasal Specimen Collection Instructions.
Instructions
- The patient should NOT eat, drink, chew gum, smoke, or vape for at least 30 minutes before collecting the specimen.
- Wash your hands and, if performing testing on someone else, wear appropriate personal protective equipment (PPE). This can include a protective gown, gloves, a face mask, and a face shield.
- Label the test tube with full name, date of collection and one other unique identifier such as date of birth or Health Card Number.
- Failure to provide this information may result in testing disqualification or delay.
- Complete all fields of the COVID-19 and Respiratory Virus Test Requisition.
- Failure to provide this information may result in testing disqualification or delay.
- Unwrap the swab from its package being careful to only hold the distant end of the swab shaft opposite the soft swab tip.
- Collect the specimen following the procedure illustrated above.
- Place the swab, soft tip first, into the test tube.
- Break the swab shaft evenly at the intended breakpoint line.
- Do not bend the swab shaft instead of breaking it.
- Reseal the test tube cap, twisting tightly.
- Failure to recap the test tube properly may result in leakage and testing disqualification.
- Place the specimen in the main compartment of the biohazard bag.
- Place the fully completed test requisition in the outer side pocket of the biohazard bag so that it is not exposed to the specimen.
- Wash your hands and confirm how to process the test to the lab.
- If collected at home, the specimen should be kept at room temperature and dropped off within two hours of collection to a designated drop off site.
- If the specimen was collected on site by a health care professional, it should be stored and transported at 2-8°C. If transport to the laboratory will be delayed beyond 72 hours, specimen should be frozen at -70°C or below and shipped on dry ice.
Storage of unused kits
Unused kits should be stored at 2-25°C until used. Improper storage will result in a loss of efficacy. Kits should only be stored up until their expiry date.
Some collection kits may have expiration dates extensions beyond the manufacturer label. The list of expiry extensions for COVID-19 testing products is available through the Ontario Health COVID-19 Health System Response Materials page.
Limitations
Swabs designed for oral and/or nasal collection should be preferentially used over swabs designed for nasopharyngeal collection (see PHO’s COVID-19 PCR Collection Kits page for details). Caution and clinical judgment may be advised when swabbing individuals with recent facial trauma, severe epistaxis, and/or significant abnormality of the nasal anatomy.
Other Resources
- Johns Hopkins Medicine. How to obtain a nasal mid-turbinate (NMT) swab for COVID-19 [video recording on the Internet]. Baltimore, MD: Johns Hopkins University; 2020 [cited 2021 Feb 01]. 1 min.
- Centers for Disease Control and Prevention. Nasal mid-turbinate (NMT) specimen collection steps [Internet]. Atlanta, GA: Centers for Disease Control and Prevention; 2020 [cited 2021 Feb 01].
Don’t have a MyPHO account? Register Now

