
Syphilis – Serology
Consistent with O. Reg. 671/92 of the French Language Services Act, laboratory testing information on this page is only available in English because it is scientific or technical in nature and is for use only by qualified health care providers and not by members of the public.
This page provides serological testing information for syphilis at Public Health Ontario (PHO). The causative agent of syphilis is Treponema pallidum. For information regarding other testing options, refer to the following PHO webpages:
Updates:
- Addition of an interpretation table.
Testing Indications
Syphilis serology testing is indicated for routine diagnosis of suspected syphilis infections and treatment monitoring of syphilis cases. Screening is recommended for individuals presenting with risk factors, and pregnant people in their first trimester or at the first prenatal visit. Repeat screening in pregnancy should be considered for individuals with ongoing or high risk. For more information on syphilis screening, refer to the Canadian Guidelines on Sexually Transmitted Infections.
If ordering as part of a prenatal screening, see Prenatal Serology. For initial syphilis testing on newborns, it is recommended that maternal serum also be submitted with a separate requisition.
Acceptance/Rejection Criteria
- Donor testing is not available through PHO’s laboratory. Specimens from patients being screened as potential donors (e.g. organ, tissue, cells, fertility, etc.) should be referred to a laboratory that performs donor screening assays. Specimens received for donor screening at PHO’s laboratory will be rejected.
- Specimens received more than 7 days post collection will not be tested.
- Cord blood samples are not acceptable.
Specimen Requirements
| Test Requested | Required Requisition(s) | Specimen Type | Minimum Volume | Collection Kit |
Syphilis |
Serum |
1.5 mL |
Serum Separator Tubes (SST) |
Submission and Collection Notes
Complete all fields of the requisition form, including:
- Test(s) requests and reason for testing
- Patient setting, specimen type and site
- Relevant clinical information
Label the specimen container(s) with the patient’s first and last name, date of collection, and one other unique identifier such as the patient’s date of birth or Health Card Number. For additional information see: Criteria for Acceptance of Patient Specimens. Failure to provide this information may result in rejection or testing delay.
Submit centrifuged serum separator tubes (SST) for serological assays.
One FULL 5 ml SST is required for testing a combination of hepatitis viruses, HTLV, syphilis and rubella. If a full tube cannot be drawn, submit two tubes.
Do NOT submit glass tubes.
Limitations
- Heat-inactivated, hemolysed, icteric, lipemic or microbial contaminated serum is not recommended for testing.
- The screen test may be falsely negative in early infection.
- Liquid anticoagulants may have a dilution effect resulting in lower concentrations for individual patient specimens.
Storage and Transport
Place specimen tube in biohazard bag and seal. Place completed General Test Requisition in the pouch at the front of the biohazard bag.
Specimen tubes should be stored at 2-8°C following collection and centrifugation, and should be shipped to PHO’s laboratory on ice packs within 3 days of collection. If delayed shipping is anticipated, remove serum from clot and store frozen at -20°C or colder, and ship on dry ice.
All clinical specimens must be shipped in accordance to the Transportation of Dangerous Good Act.
Test Frequency and Turnaround Time (TAT)
Syphilis screening is performed daily Monday to Friday.
Syphilis confirmatory testing is performed daily Monday to Friday.
Turnaround time is up to 3 business days from receipt at PHO’s laboratory for non-reactive specimens and up to 6 business days for reactive specimens.
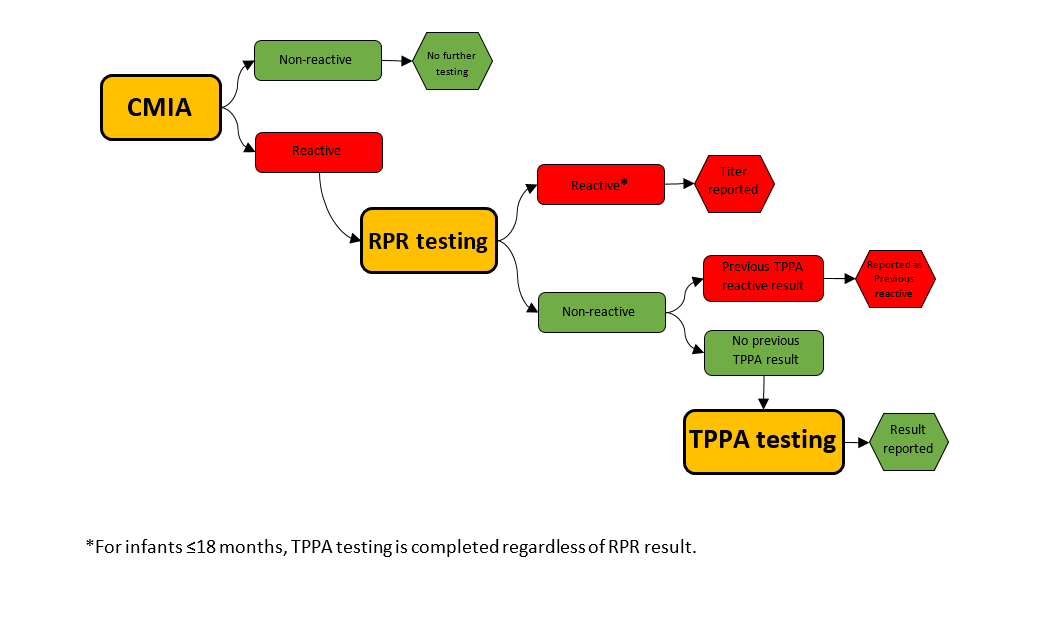
PHO follows a reverse algorithm for syphilis testing.
- Syphilis Screening: Chemiluminescent microparticle immunoassay (CMIA) – a qualitative immunoassay that detects treponemal antibodies (IgG and IgM) to Treponema pallidum. This test does not distinguish between IgG and IgM.
- Syphilis Confirmatory:
- Rapid plasma reagin (RPR) - a semi-quantitative flocculation assay that detects non-treponemal antibodies to cardiolipin-lecithin- cholesterol (Reagin antibodies).
- Treponema pallidum particle agglutination (TP.PA) - a qualitative gelatin particle agglutination assay that confirms antibodies (IgG and IgM) to Treponema pallidum. This test does not distinguish between IgG and IgM. TP.PA reactivity is used as an aid in the diagnosis of current/past syphilis infection.
Algorithm
Diagram 1. Syphilis serology testing algorithm
Interpretation
Syphilis serology results must be interpreted with the clinical, treatment, and exposure history of the individual. Syphilis staging cannot be done with serology results alone. The following table provides interpretations and recommendations for the most common results.
Syphilis Serology Result Interpretations
This table organizes case results in four subject headings (labelled from left to right): Serology Screening Test CMIA, Confirmatory Test RPR, Confirmatory Test TPPA, and Possible Interpretation and Recommendations. Case results are reported as per the headings and collated in table form for easier viewing of the interpretation.| Serology Screening Test (CMIA)1 | Confirmatory Test (RPR) | Confirmatory Test (TPPA)1 | Possible Interpretations/ Recommendations |
|---|---|---|---|
|
Non-reactive |
Not tested |
Not tested |
|
|
Reactive |
Reactive (titer) |
Not tested |
|
|
Reactive |
Reactive (titer) |
Reactive/ |
|
|
Reactive |
Non-reactive |
Reactive/ |
Patients ≤18 months
|
|
Reactive |
Non-reactive |
Non-reactive |
Patients ≤18 months
|
|
Reactive |
Non-reactive |
Indeterminate |
|
1Maternal antibody transfer can be present in infants for up to 18 months.
2https://cps.ca/en/documents/position/congenital-syphilis
Don’t have a MyPHO account? Register Now

